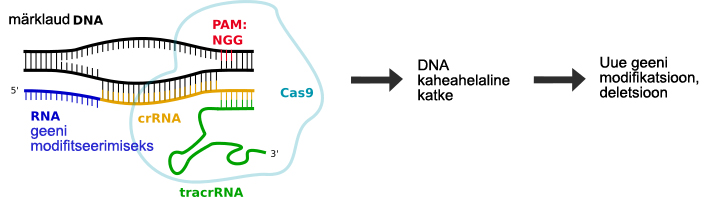
There are more than 500 different types of human cancers. Wouldn’t it be wonderful if scientists could develop cures for all of them? Scientists believe that CRISPR gene-editing can be used to cure some cancers. CRISPR (an acronym for clustered regularly interspaced short palindromic repeats) is a way of targeting a specific bit of DNA inside a cell which can then be gene-edited to change such bit of DNA. CRISPR has also been used for other purposes, such as turning genes on or off without changing their DNA sequence.
Recent research has found a link between CRISPR gene-editing and mutated cancer cells. Scientists believe that a further understanding of this link can identify a group of genes which should be monitored for mutations when cells are subjected to the CRISPR gene-editing method. Although CRISPR gene-editing holds promise for cell repair, the application of CRISPR gene-editing, which is meant to identify and correct damage in cells, can also cause damage to cells in a controlled manner. Such damage activates a protein, p53 (“also known as the guardian of the genome”), which helps repair damaged DNA.
P53 is a transcription factor, which is a protein that regulates the rate at which DNA is transcribed into RNA. These transcription factors bind to regulatory sequences in proteins, thus changing the shape of DNA, ultimately making them the most vital form of gene regulation. Transcription factors include many proteins but exclude RNA polymerase, which pries two strands of DNA apart and joins two strands of DNA together (Campbell, 280). P53 works by sliding along the damaged DNA, seeking a critical site to which it attaches and then sends a message to halt cell division until the DNA is repaired. In other words, p53 acts as a checkpoint in the cell cycle, preventing cell from proceeding though the G1 and G2 phases of the cell division cycle. In mice, the same exact transcription factor exists; those that lacked the Trp53 gene developed tumors at a far faster rate than those with the functioning gene.
By using CRISPR technology to damage DNA at the same cite at which DNA damage occurs, scientists are able to identify the protein responsible for cellular proliferation. If damage to the cell is too severe then p53 triggers apoptosis (the death of cells which occurs as a normal and controlled part of an organism’s growth or development) so that the damaged cell is destroyed. However, sometimes p53 is itself damaged which prevents such protein from binding to the damaged DNA in order to repair it or otherwise signaling destruction of the cell. When this occurs, the damaged cells multiply and grow, resulting in tumors. Scientists have found alterations in p53 in more than half of all cancers and thus, consider p53 the most common event in developing cancer.
New studies show that p53 inhibition can make CRISPR more effective thus, counteracting “enrichment” (the process of purifying cells for downstream applications such as qRT-PCR, cell polarizations ex vivo, or to enrich cells for use in a flow cytometry experiment) of cells with p53 mutations which has been observed to occur in cell cultures when such cells have been subjected to CRISPR. In other words, there is in vitro evidence that CRISPR technology causes harmful p53 mutations to be more prevalent in the population that has been subjected to the CRISPR technique. These findings suggest that there is a group of genes that should be monitored for mutations when the CRISPR gene-editing method is applied to cells.
Cancer is a devastating disease that has taken the lives of many people. Members of my family have suffered and lost their battle to cancer (most recently my dear aunt this past weekend). CRISPR presents the possibility of finding cures to cancer which are specifically designed to target the particular genetic mutations that are unique to each individual. Perhaps, the cure to cancer will be achieved sooner than we realize, although clearly not soon enough.
Works Cited:
Reece, Jane B, and Neil A. Campbell. Campbell Biology. Boston: Benjamin Cummings / Pearson, 2011. Print.


Leave a Reply