You read that correctly! Researchers at Rice University in Houston, Texas alongside Jeffrey Hartgerink have made a significant advance in injury treatment, illness education, and drug candidate by testing the self-assembling abilities of 3D printed nanofibrous multidomain peptide hydrogels, referred to as “hydrophobic sandwiches.”
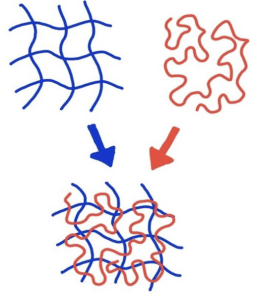
The main goal of Hartgerink’s team was to create a structure that could house cells and help them grow tissue by 3D printing the peptide ink. The printing allows researchers to recreate the complexity of biological structures due to their soft and flexible tissue-like feel, making this a major scientifical discovery and advantage. Hartgerink and his team describe their printed peptides as “hydrophobic sandwiches” due to their design, flexibility, and behavior. The peptides were printed to have one hydrophobic side and one hydrophilic side, allowing them to flip on top of each other when placed in water and resemble sandwiches. Like we learned in AP Biology, the hydrophillic qualities of one side will attract water, and the hydrophobic qualities of the other will repel water. Hydrophobic molecules repel water because they are nonpolar molecules, so they are not attracted to water, which is polar. Once the “sandwiches” were stacked after flipping in the water, they formed the hydrogels which can be vital to tissue engineering and wastewater treatments.
The multidomain peptides have already been utilized due to their self-assembling nature for regenerating nerves, treating cancer, healing wounds, and encouraging tissue development throughout the body. Rather than only focusing on this aspect of the peptides, Adam Farsheed, a lead author in Hartgerink’s study, wanted to specifically highlight the fact that these peptides are an ideal 3D-printing ink choice due to their self-assembling nature. When testing the “sandwiches,” Farsheed took a unique, brute-force approach to add more of the material, rather than chemically modifying it, to test its function and ability to reassemble itself after deformation. He proved that adding more peptide material lets the peptide reassemble and heal itself extremely well after being deformed. This discovery will make the hydrogels an ideal candidate for scientific and medical usage.
Through continued testing, he was also able to confirm that the peptides behave differently depending on their charge. The peptide cells with a negative charge tended to ball up on the substrate of the experiment and the positively charged cells spread out and started to mature on their own. Farsheed has confidently stated that their findings will allow the group to “control cell behavior using both structural and chemical complexity.” Both Hartgerink and Farsheed have made incredible contributions to the world of science through their studies using 3D-printed peptide hydrogels.


Leave a Reply