What is CRISPR-Cas9? CRISPR-Cas9 (Clustered, Regularly Interspaced Short Palindromic Repeats) is a powerful technology that allows geneticists to modify or “edit” parts of the target genome by adding or removing whole sections of the DNA sequence. Currently, CRISPR is the most versatile and accurate DNA modification tool globally. This tool allows scientists to fix flaws in most organisms’ DNA and has minimal risk of off-target damage.
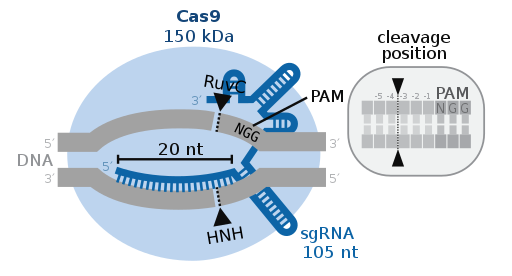
CRISPR-Cas9 has two main molecules that carry out the change in DNA, an enzyme called Cas9, and a piece of RNA called guide RNA (gRNA). The Cas9 enzyme locates the target area and can cut the DNA in a specific location in the genome so that small pieces of DNA can either be added or removed. The gRNA is made of a small piece of a lab-designed RNA sequence, roughly 20 bases long, located within the RNA scaffold. To ensure that the Cas9 enzyme cuts at the right point in the genome, the scaffold binds to the DNA, and the lab-designed sequence pilots the Cas9 enzyme to the correct location. The gRNA has RNA bases that match those of the target DNA sequence in the genome. The Cas9 enzyme follows the gRNA to the specific area and makes a precise cut across both strands of the DNA. During this stage, the cell recognizes that the DNA has been damaged and will try to repair itself. Scientists use this DNA repair system to add or remove changes in one or multiple genes. This technology is consistent with our most recent AP Biology Unit, DNA Replication, and Gene Expression/Replication.
In my opinion, CRISPR-Cas9 is an incredible technology as it has so many practical applications. The future of this technology has potential in many diverse fields such as genetic engineering, bioengineering, and molecular biology, among other areas of study.
The technology has been tested on dogs with Duchenne Muscular Dystrophy, a gene mutation adversely affecting muscle proteins. In this case, a CRISPR gene-editing treatment demonstrated promising signs of permanently fixing the genetic mutation responsible for this disease, which in humans, affects approximately 1 in 3,500 male births worldwide. The mutation prevents an organism from producing an appropriate level of functioning dystrophin which causes muscles to be weak and not respond efficiently. Researchers at the University of Texas Southwestern found that gene editing restored the functioning dystrophin levels in the dog’s muscles and heart tissue. The increase in the dystrophin levels would need to be more significant for it to work in humans, but researchers have been making substantial progress in advancing this developing CRISPR-Cas9 technology.


Leave a Reply