Telomerase. They know what it is. They know what it does. They know it is involved with the formation of malignant tumors. Yet for years, cancer researchers could not figure out a way to curb telomerase activity. Not until recently, when a group of researchers at the University of California, Santa Cruz discovered an important structural component of telomerase that could lead to the development of new and more efficient cancer treatment medications.
But first things first: what even is telomerase? To understand the role of telomerase, we must first understand what a telomere is. Analogous to the “plastic tips of shoelaces”, telomeres are located at the tips of chromosomes to keep the ends of DNA from “fraying”, consisting of the repetition of the same nitrogenous base sequence over and over again. In humans, this base sequence is TTAGGG.
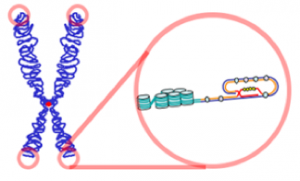 (Source: https://en.wikipedia.org/wiki/Telomere#/media/File:Telomere.png)
(Source: https://en.wikipedia.org/wiki/Telomere#/media/File:Telomere.png)
The sequence can be up to 15,000 base pairs long; however, each time a cell divides, the telomeres get shorter and shorter until they become they become too small to divide again. That is when the telomerase comes in; it adds nucelotides to the telomere to prevent it from becoming senescent, or at least prolong the cell’s life span.
Sounds like a good thing, right? Not when the telomerase gets out of control and does not allow for cells to die, causing a huge growth of cells that eventually evolve into malignant tumors.
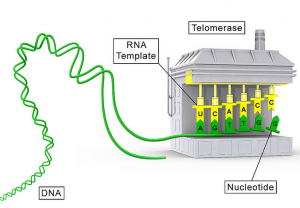
What makes it hard for scientists to combat excessive telomerase activity is due to the enzyme’s unique and complex structure. In addition to its sophisticated quaternary structure, telomerase also has an RNA template that allows the telomerase to make the DNA bases (TTAGGG) for the telomere.
(Source: https://vi.wikipedia.org/wiki/Telomerase#/media/File:Telomerase_illustration.jpg)
Researchers at UC Santa Cruz determined the structure of the RNA binding domain of telomerase and how the template border is dependent on how the protein and RNA components interact with each other. Understanding this interaction can help scientists develop cancer medications that more specifically inhibit telomerase. This is the first major advancement in telomerase research since November of 2010 when biochemists at UCLA created an unprecedented 3D model of telomerase’s RNA structure.
While this discovery is a major step forward in cancer treatment research, some experts have their reservations against finding methods of inhibiting telomerase altogether.
However, regardless of the controversy surrounding telomerase inhibition in cancer treatment, this discovery will be useful in coming up with tactics to prevent aging, and improve treatments in other medical fields, such as burns, bone marrow transplants, and heart disease.
What do you think? Leave a comment below!


Leave a Reply