What is Duchenne Muscular Dystrophy?
Duchenne muscular dystrophy, DMD, is an X-linked recessive disease caused by defects in the gene that makes the dystrophin protein. This particular gene is made of 79 exons, and the defects can occur on any of them. These defects lead to degeneration of skeletal and heart muscle, forcing patients to rely and wheelchairs and respirators. Without a cure, most people with the disease die by the age of 30. So, the question becomes, how can we find a cure?
What is Precision Editing?
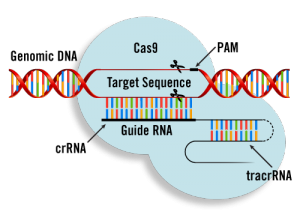
CRISPR technology has advanced tremendously in the past several years, with each study building off the last. CRISPR technology has the capability to cut out segments of DNA, but with the risk of cutting out too much or the wrong parts. Thus, it is crucial that the cutting be as precise as possible. The CRISPR-Cas9 gene-editing tool, uses an RNA strand to guide the Cas9 enzyme along the DNA strand, skipping over important “healthy” DNA and leading the enzyme to cut a specific portion of DNA.
How do DMD and Precision Editing Connect?
Dr. Olsen is Co-Director of the Wellstone Muscular Dystrophy Cooperative Research Center, a lab in which a team has been working to apply precision editing to DMD. The method uses one single cut of DNA along strategic points and is less intrusive than other methods. Scientists have developed guide RNAs with the purpose of finding mutation “hotspots” along the dystrophin gene. The RNA strand guides the Cas9 enzyme to 12 regions where most DMD mutations have been found. According to the article, “the new strategy can potentially correct a majority of the 3,000 types of mutations that cause DMD.” Wow! In a recent study using this method, these RNAs helped rescue cardiac function to near-normal levels in human heart muscle tissue.
Why is it Important?
The new study demonstrates eliminating abnormal splice sites in human DNA can correct a wide range of mutation. In the case of DMD, the splice sites that were removed using CRISPR technology instruct the genetic machinery to build abnormal dystrophin molecules. Once these sites are removed, an improved dystrophin protein was observed. Even more fascinating, correcting only half of the damaged cells restored cardiac function to a healthy level. Does this sound fascinating? If you answered yes, click here to learn more!
What Does the Future Hold?
The strategy of single-cut editing may be useful for treating other single-gene diseases. News of such prospects has generated a great deal of hope for patients. Much more research is needed before CRISPRCas9 can be used on human patients. Labs and researchers around the world are working to perfect this method so that it can get federal government approval and move to the next stage – human trials. As research progresses, it will be faced with backlash from some who believe DNA should not be altered and that the technique is too risky and support from those who believe this new technique could save lives. Which side do you fall on?



Leave a Reply